Aerospace & Mobility
Materials that contribute to human progress, taking us higher and further.
Find outENGINEERING
Combining innovation and high requirementsstandards to create innovative materials for solutions appropriate to your needs.
Find outComposites Manufacturing
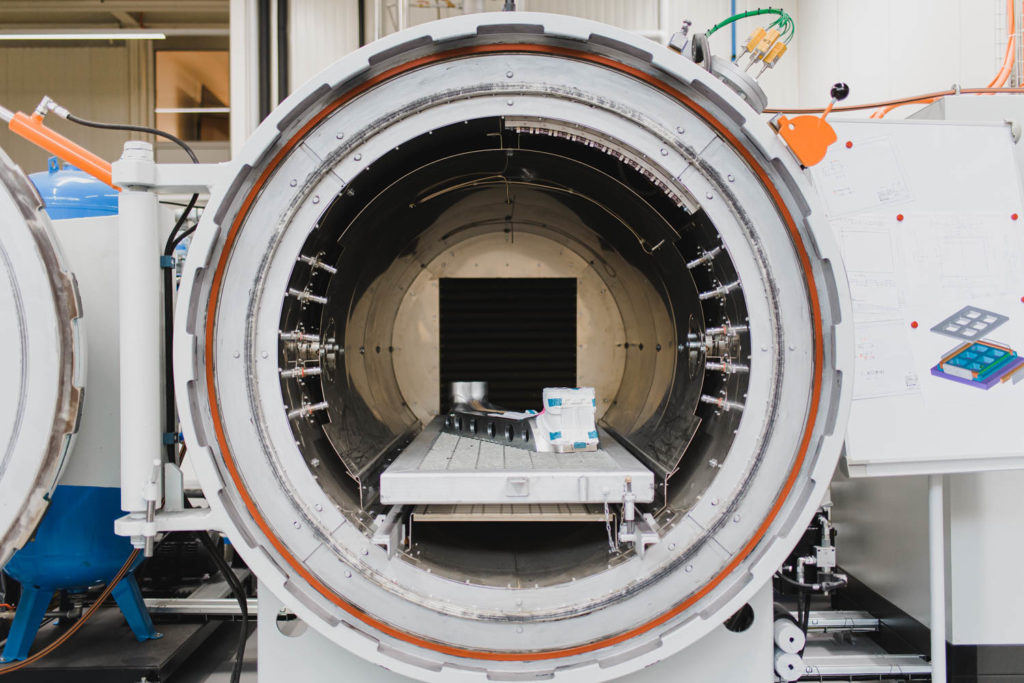
Harmonising human expertise with machine precision to shape composite materials into suitable, coherent forms.
Find outADDITIVE MANUFACTURING
Unlocking the potential combining flexibility and cost-effectiveness for total freedom in shapes and surfaces.
Find outQUALITY MANAGEMENT AND ASSURANCE
Overseeing processes and managing risks while remaining true to the company’s values of innovation and humanism.
Find out